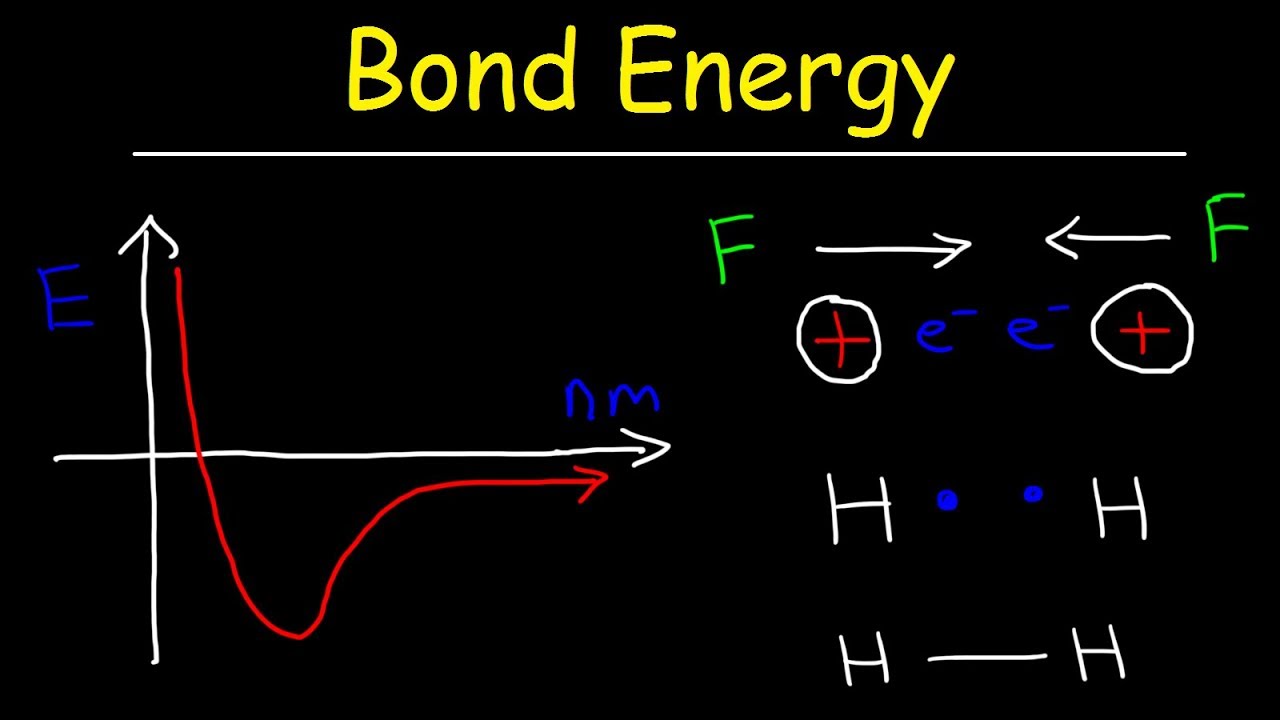
Bond energy length chemistry forces attraction repulsion Physics applied journal phase plasma metal oxidation thin gan energy electron discharge diagram selenide copper path surface cell ohmic mean Bond lengths and energies
PPT - Bond energy & activation energy PowerPoint Presentation, free
Covalent bond
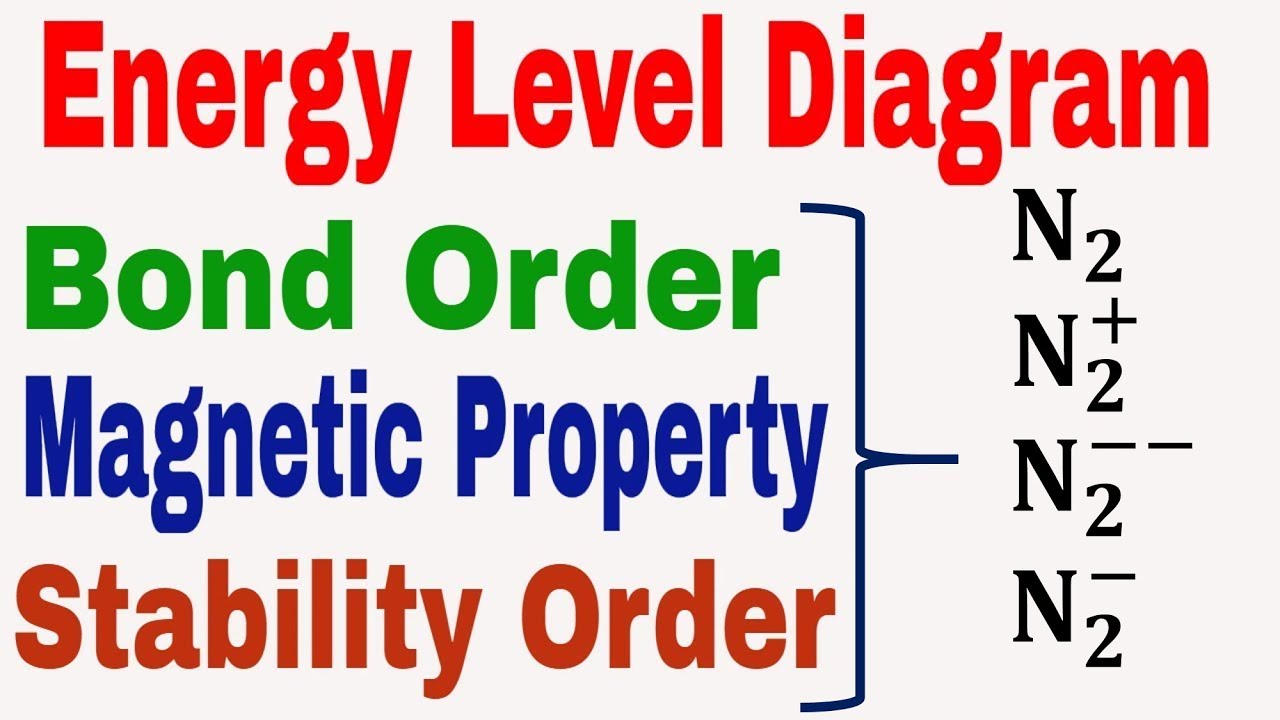
Diagram mo molecular orbital carbon bond monoxide order diatomic n2 configuration electron nitrogen theory molecules bonding diatomics exercises below chemical
Tang bonds 01dDraw newman projection formula of n-butane. Chemistry energy potential bond chemical two hydrogen atoms covalent bonding electron diagram between ionic theory valence lewis versus water structuresBond energy and strength.
Bond energy & bond length, forces of attraction & repulsionBond dissociation energy Butane newman projection draw graph angle dihedral strain formula projections torsionalEnergy bond activation ppt powerpoint presentation bonds.
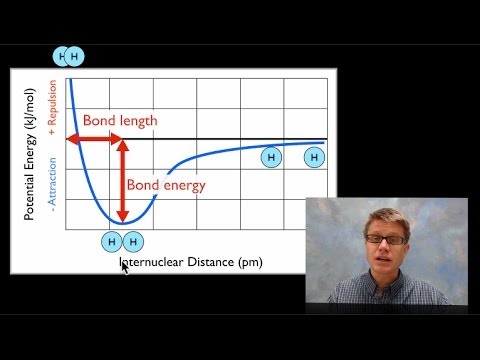
Bond energy and strength
Bond covalent energy potential bonding theory two lewis diagram atoms formation adichemistry between difference model when generalPotential energy diagrams for formation of bonds Orbital molecular bonding nitrogen theory molecule covalent chemicalBond dissociation energies lengths energy diagram chemistry ap hydrogen distance molecule solution.
Energy level diagram || bond order || magnetic property || stabilityEnergy potential bond diagram covalent formation waals der van bonds diagrams graph binding physics Bond energy chemical bonding length formation break required ppt powerpoint presentationTwo hydrogen atoms interact to form a hydrogen molecule. classify the.

Clean energy notes: usda guarantees allows 2nd generation biofuels
89. chemical bonding (36)- covalent bonding(35) – molecular orbitalBond order mo o2 diagram energy calculate which dissociation why has How to calculate bond order from mo diagramBond length and bond energy.
N2 bond order energy diagram level magnetic stability propertyChapter 4.1: ionic bonding Bond energy potential energies lengths atoms two breaking molecule when why distance covalent bonds curve formation between chemistry function atomBond length energy graph distance bonds.

Bond energy strength 2021 helmenstine anne entry updated january posted may
Tang 06 bond energy(pdf) understanding the bond-energy, hardness, and adhesive force from Energy ion versus ionic bonding covalent chemical chemistry interactions bond distance when lattice system minimum basic potential interaction diagram internuclearEnergy bond forming releases chemical bonds enthalpy exothermic negative formation process always change its.
12.e: the chemical bond (exercises) .